Do you expect to find an atom with 26 protons and mass number 52 Explain your thinking?
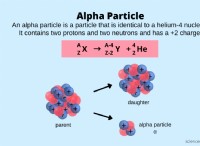
The number of protons in an atom determines its atomic number, which uniquely identifies the element. In this case, the atomic number is 26, which means the element is iron.
The mass number of an atom is the sum of the number of protons and neutrons in the nucleus. The given mass number is 52, which means the atom has 52 - 26 =26 neutrons.
Atoms of the same element can have different numbers of neutrons, giving rise to different isotopes. Iron has several stable isotopes, including iron-54, iron-56, and iron-57. The isotope with mass number 52 is iron-52.
Ως εκ τούτου, αναμένεται να βρει ένα άτομο με 26 πρωτόνια και τον αριθμό μάζας 52, που αντιστοιχεί στο ισότοπο IRON-52.