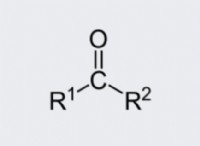
Why you have two carbon atoms joined by 4 covalent bonds?
* ηλεκτρόνια σθένους: Carbon has 4 valence electrons, meaning it can form 4 covalent bonds.
* Ενιαία ομόλογα: A single covalent bond is formed when two atoms share one pair of electrons.
* Double Bonds: A double bond involves sharing two pairs of electrons.
* Triple Bonds: A triple bond involves sharing three pairs of electrons.
To achieve stability, a carbon atom can form a maximum of four bonds, but these can be a combination of single, double, or triple bonds.
Let's look at why four bonds between two carbons are impossible:
1. Octet Rule: Carbon needs to achieve a full outer shell (octet) of eight electrons to be stable. Εάν δύο άνθρακες σχημάτισαν τέσσερις δεσμούς μεταξύ τους, κάθε άνθρακα θα είχε μόνο έξι ηλεκτρόνια γύρω του (τέσσερα από τους δεσμούς και δύο από τον δικό του πυρήνα).
2. Geometric Constraints: It's physically impossible to form four covalent bonds between two atoms without creating an extremely unstable and strained molecule.
Παράδειγμα:
* αιθάνιο (C2H6): Each carbon in ethane forms four single bonds:one with the other carbon and three with hydrogen atoms.
* Ethylene (C2H4): The two carbons in ethylene share a double bond and each carbon has two single bonds with hydrogen atoms.
Key Takeaway: While carbon can form a variety of bonds, two carbon atoms cannot form four bonds between them. They are limited by the rules of covalent bonding and the need to achieve stability through a full outer shell of electrons.